Water Quality Control: The DIAMSENS Technology
- Home
- Technology




Our sensors are part of the large family of electrochemical sensors. This measurement method is very much used for water quality monitoring. Our sensors inherit the recognized characteristics of the method, including sensitivity and accuracy.
The choice of diamond allows us to satisfy the demands of users for more durability and less maintenance. Since diamond – even “lab grown” – is an expensive material, we opted for a material made of diamond nanocrystals, which can be manufactured much faster than monocrystals. We use proven, automated microelectronics techniques to assemble the sensors in a cost-effective way.
Water quality: electrochemical sensors without any compromise on performance
How does an electrochemical sensor work?
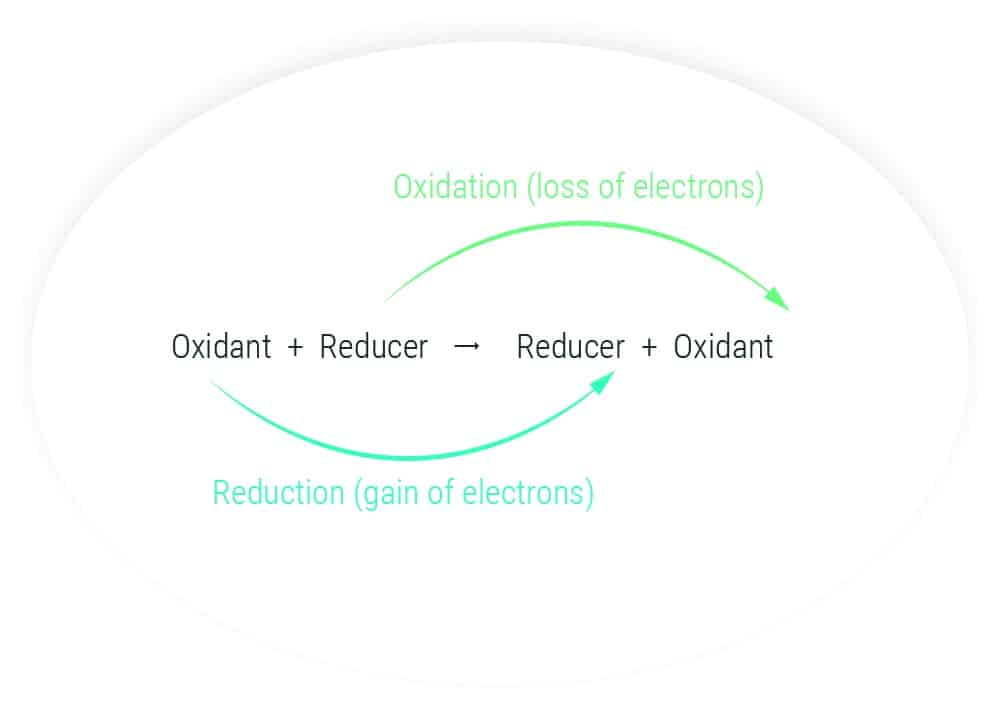
Electrochemistry is first and foremost an oxidation-reduction chemical reaction, which involves an exchange of electrons between two species: a strong oxidizer (electron-hungry species) and a strong reducer (electron-supernumerary species).
A complete example can be seen in the video.
In an electrochemical sensor, one of the two species is replaced by an electrode – a metal pellet – whose potential is modified to make it an electron acceptor or donor. The intensity of the electron exchange is proportional to the concentration of the species in solution with which the electrode interacts.
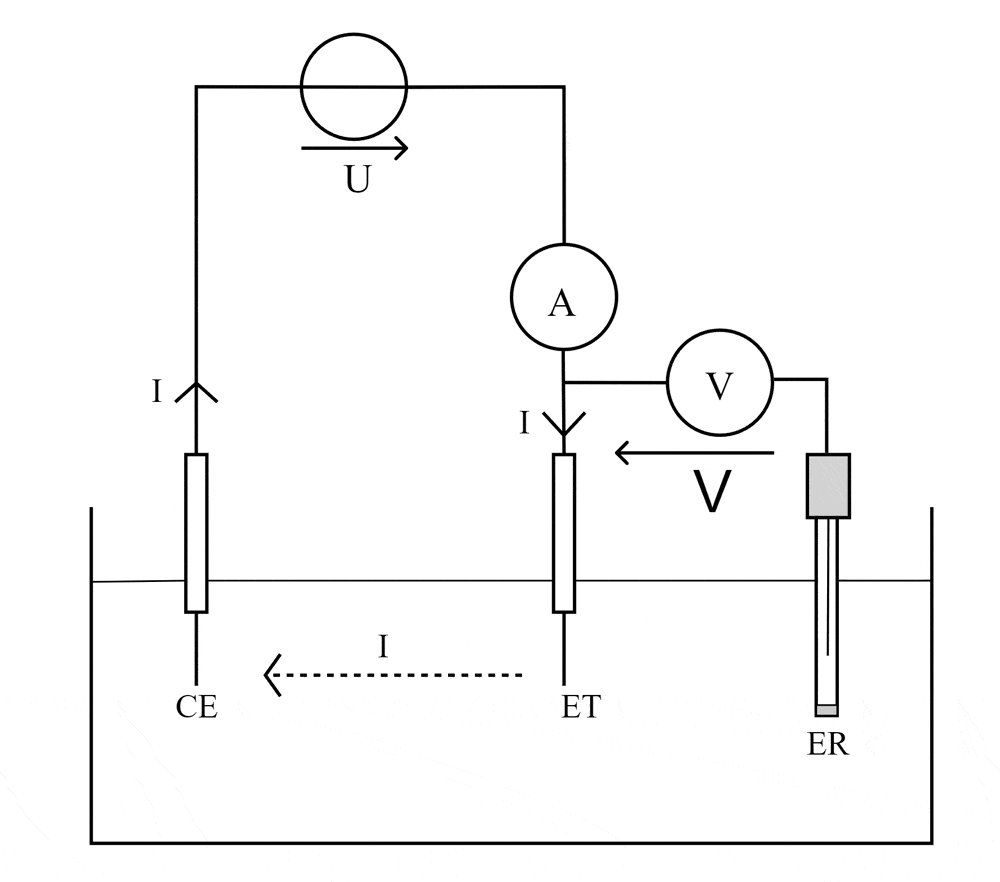
We can observe/measure either an electric potential difference (which gives a picture of the energy needed to activate an electron exchange) or an electric current (which represents the dynamics of the exchange). To do this, we will use two or three electrodes respectively that we will polarize to cause the desired oxidation-reduction reaction. High-performance instrumentation will then collect the generated signal (which remains relatively weak).
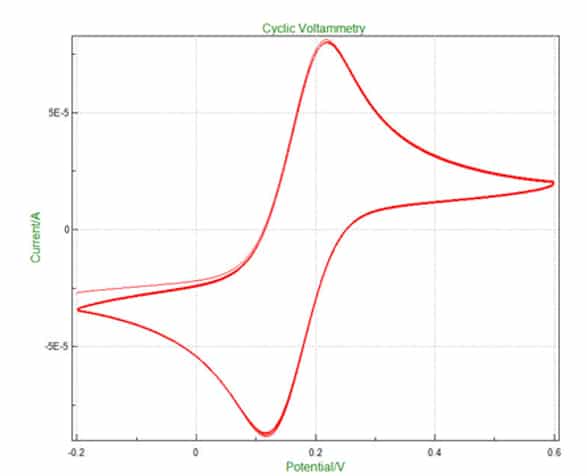
Most of the DIAMSENS sensors are of the amperometric type. A voltage is applied between two electrodes (the so-called “reference” electrode and the so-called “working” electrode) and the resulting current is observed on a third electrode (called the “counter electrode”). With a voltage scan, voltammetry curves can be plotted in the characteristic shape known as the “duck”.
In this case, it is the height of the peak that is proportional to the desired concentration. The mediating potential between the two peaks is used to identify the species being measured.
What are the quality criteria of an electrochemical sensor?
In order to compare sensitive materials with each other, regardless of the final measurement that is targetted, a series of characterizations is generally followed. Of particular note are:
The width of the scan window:
- The wider the window, the more different reactions will be detected.
- The flatter the curve, the less the measurement will be disturbed by parasitic reactions.
- The lower the base current, the lower the minimum detectable current will be, and thus the better the sensor’s “detection limit”.
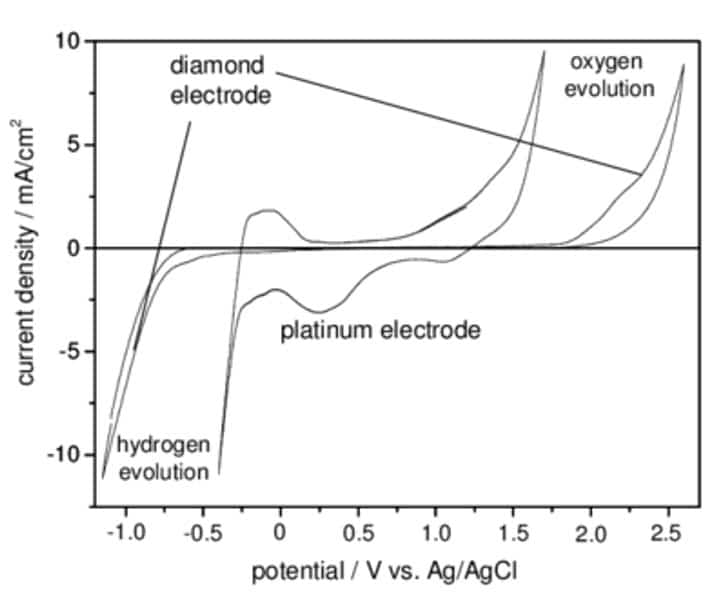
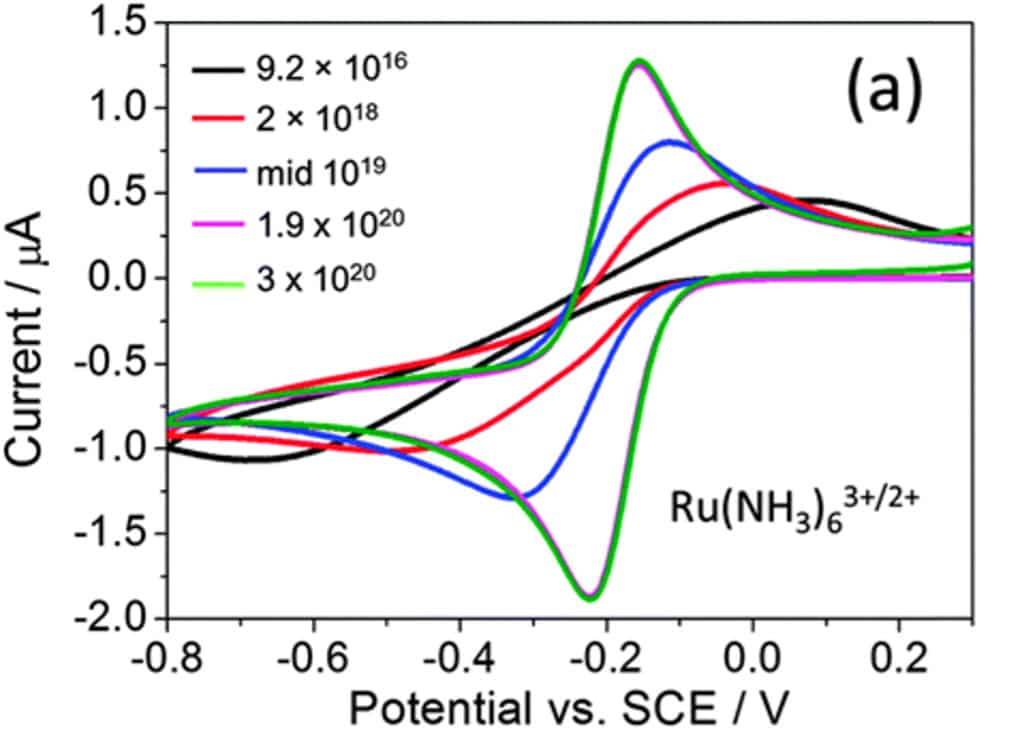
The shape of the I=f(V) curves in a reference medium, e.g. rhutenium hexaamine:
- The higher the current peak, the better the sensitivity,
- The closer the positive and negative peaks are to zero, the better the surface reactivity, and therefore the sensitivity of the measurement.
As an example, the curves opposite show the evolution of the characteristics according to the concentration of a doping agent in the base material.
How does diamond perform?
Boron-doped diamond, the benchmark for diamond sensors, has excellent performance according to these criteria. Our diamond material version behaves just like the reference diamond. DIAMSENS has patented a generic structure of electrodes, associated with manufacturing processes, which makes it possible to achieve the best performance while optimizing the production costs of an entire electrode and then a sensor.
A sensor permanently immersed in water degrades due to two phenomena:
- An ageing of its structure: water is a highly oxidizing medium that will interact with the materials used to build the sensor. Water can deform the structure of the sensor and/or dissolve any on-board reagents, which will have an impact on the analytical performance of the sensor.
- Fouling of the mating surfaces, with a direct impact on the sensitivity of the surface.
Diamond is the most chemically inert material, it will not structurally deform or change.
The CEA has patented a process for self-cleaning dirty surfaces using electrical methods, which is both efficient and relatively energy-efficient. In this way, the sensor is regenerated and regains its original sensitivity.
DIAMSENS monitors the progressive clogging of the electrodes and only activates this self-cleaning process when the accuracy of the measurements is at stake. The energy expenditure is thus minimized
Sensors that are easy to integrate into water quality monitoring equipment
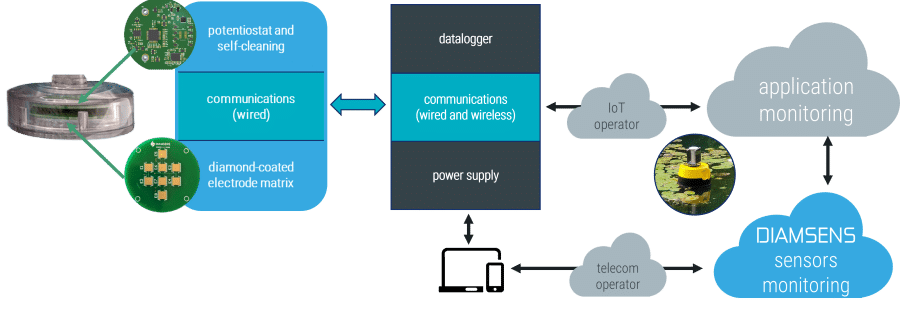
The electrodes and the electronic measuring device are supplied encapsulated in a sealed housing. The dimensions depend on the number of sensors chosen. For a typical case (4 to 6 sensors) the case will have a diameter around 60mm and a height around 15mm.
Regardless of the number of sensors, a single cable connects the housing to the measuring station, transmitting the data and receiving the power supply. For industrial applications with a remote measuring station, an RS485 interface is available.
DIAMSENS provides the software libraries that allow the measuring station to easily control the sensors and download the measurement results.